Posted on September 3, 2014 in Product Liability
Risperdal is an antipsychotic drug approved to treat people who suffer from schizophrenia and bipolar disorder. It has been associated with very serious and sometimes permanent side effects. While adverse outcomes may happen with any medication, pharmaceutical companies have a duty to disclose those risks to federal regulators and the public. Unfortunately, that disclosure did…
Posted on August 20, 2014 in Product Liability
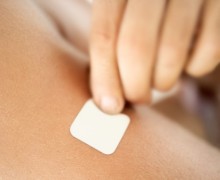
Fentanyl is a narcotic pain reliever designed to treat chronic pain. Fentanyl is marketed under the name Duragesic, Actiq, Fentora and Sublimaze, according to the National Institute on Drug Abuse. The medication is administered through injections, lozenges or adhesive skin patches that release the medication over an extended time. Unfortunately, some patients who are prescribed…
Posted on July 16, 2014 in Product Liability
Graco’s Children’s Products has recalled 1.9 million infant car seats following a months-long debate with U.S. regulators about a faulty buckle, the Lexington Herald-Leader reports. Earlier this year, the company recalled more than 4 million toddler car seats after receiving reports of children getting stuck in the seats when the buckle jammed. In some cases,…
Posted on May 14, 2014 in Product Liability
Nearly 2,000 children are treated in emergency rooms each year for injuries caused by improperly installed baby gates, according to a study recently published in the journal Academic Pediatrics. Researchers from Nationwide Children’s Hospital in Columbus, Ohio, found that between 1990 and 2010, an estimated 37,763 children under the age of seven suffered injuries after…
Posted on March 5, 2014 in Product Liability
The Food and Drug Administration recently announced it is investigating the risks associated with prescription testosterone replacements. Several recent studies have linked testosterone products to an increased risk of cardiovascular events including heart attacks and strokes. At least one consumer advocacy group says the FDA should be more aggressive and require a black box warning…